Welcome to the blog section of Biotrial, where we bring the world of medical research and paid clinical trials to your fingertips. We hope that you will find our posts both enlightening and engaging. Happy reading!

In the ever-evolving landscape of healthcare, clinical trials play an indispensable role in the development and validation of new treatments and therapies. These trials are instrumental for advancing medical knowledge and improving patient care. However,...
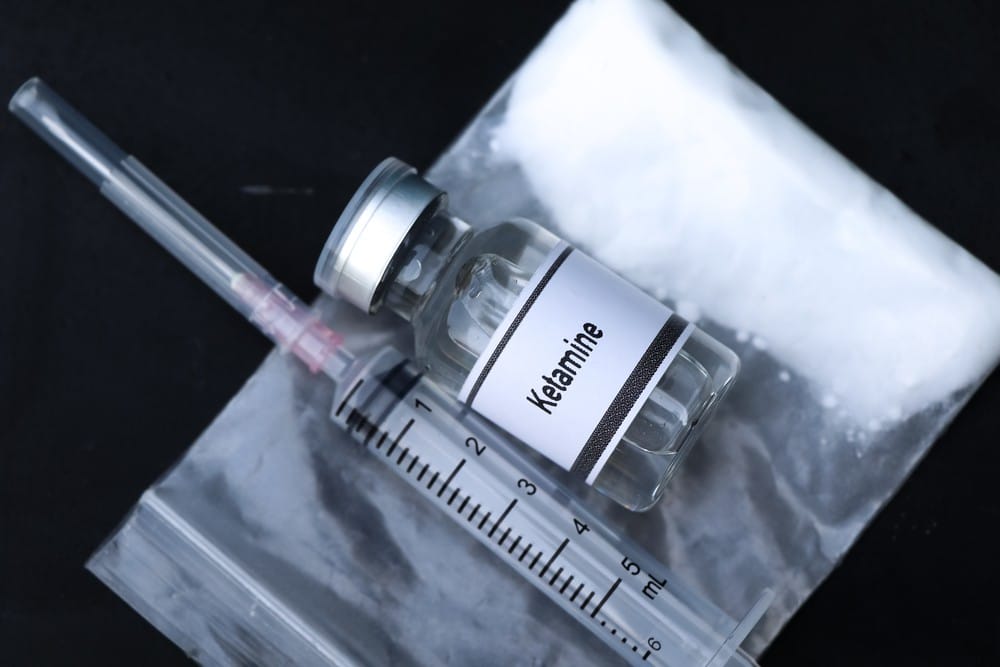
The ever-evolving world of medical research constantly presents us with innovative approaches, allowing us to delve into uncharted territories and discover new treatments. One such pioneering method gaining recognition is the Ketamine Challenge Clinical Trial,...

Clinical trials are a cornerstone of medical research, playing a pivotal role in the development of new treatments and medications. These trials serve as a bridge between the laboratory and real-world applications, ensuring that medical...

Integrating cognitive tests during clinical trials represents a pivotal stride in medical research. Cognitive testing is a method used to gauge mental processes such as memory, attention, and problem-solving abilities. It has become a cornerstone...

Clinical trials are the backbone of medical advancements, providing critical data that pave the way for new treatments to become available. Biotrial, a respected name in clinical research, contributes significantly to this intricate system. Understanding...
We need healthy men and women 18 to 80 all year round to become volunteers for our Phase I paid clinical trials. Our medical research studies compensate your time and effort. Register now to participate.

Complete the registration form and call us at 844-246-8459. Our recruiters will be happy to help you, and it will take 5 minutes of your time to know if you are eligible. Your information will be kept strictly confidential.